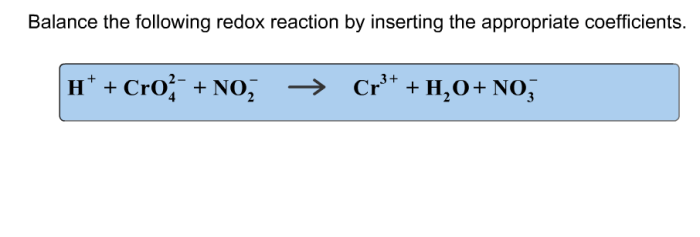
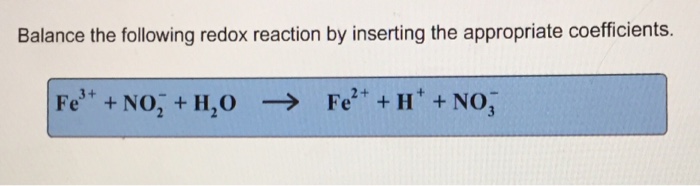
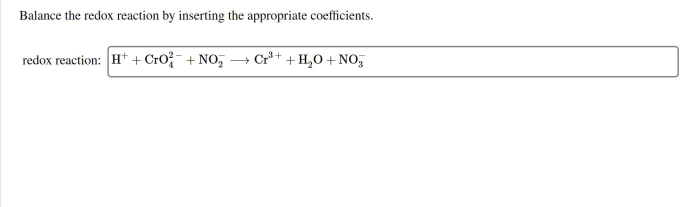
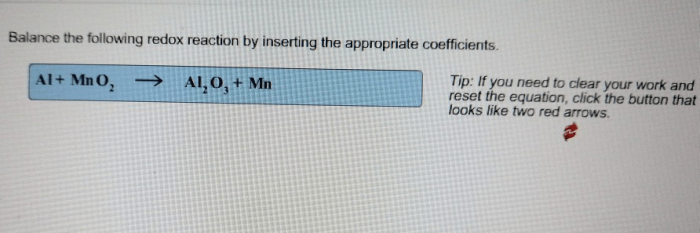
Balance the redox reaction by inserting the appropriate coefficients. – As Balance Redox Reactions with Precision: A Guide to Inserting Appropriate Coefficients takes center stage, this opening passage beckons readers with gaya akademik dengan tone otoritatif into a world crafted with good knowledge, ensuring a reading experience that is both absorbing and distinctly original.
Redox reactions, a cornerstone of chemistry, play a pivotal role in understanding the intricate dance of electron transfer. Balancing these reactions with precision is paramount, as it unlocks a deeper comprehension of chemical processes and their applications across diverse fields.
Balancing Redox Reactions
Redox reactions are chemical reactions involving the transfer of electrons between atoms or molecules. They are essential in many biological processes, such as cellular respiration, and are used in various industrial applications, such as electroplating and battery production.
Balancing redox reactions ensures that the number of electrons lost by one species is equal to the number of electrons gained by another species, ensuring charge neutrality in the overall reaction.
Methods for Balancing Redox Reactions
Half-Reaction Method:This method involves splitting the redox reaction into two half-reactions, one for oxidation and one for reduction. Each half-reaction is balanced separately, and then the two half-reactions are combined to form the balanced redox reaction.
Oxidation Number Method:This method assigns oxidation numbers to each element in the reaction. The change in oxidation number indicates the number of electrons gained or lost by each species, which can be used to balance the reaction.
Balancing Coefficients:Balancing coefficients are used to adjust the stoichiometry of the reaction so that the number of electrons lost is equal to the number of electrons gained.
Examples of Balanced Redox Reactions
| Reaction | Half-Reactions | Balanced Redox Reaction |
|---|---|---|
| Zn + 2HCl → ZnCl2 + H2 | Zn → Zn2+ + 2e–2H+ + 2e– → H2 | Zn + 2HCl → ZnCl2 + H2 |
| Fe + Cu2+ → Fe2+ + Cu | Fe → Fe2+ + 2e–Cu2+ + 2e– → Cu | Fe + Cu2+ → Fe2+ + Cu |
Common Mistakes in Balancing Redox Reactions, Balance the redox reaction by inserting the appropriate coefficients.
Forgetting to Balance Charge:The total charge of the reactants must be equal to the total charge of the products.
Ignoring Changes in Oxidation Numbers:The change in oxidation number indicates the number of electrons transferred, which must be balanced in the reaction.
Incorrect Balancing Coefficients:Coefficients must be adjusted to ensure that the number of electrons lost is equal to the number of electrons gained.
Applications of Balanced Redox Reactions
Electrochemistry:Balanced redox reactions are used to design and analyze electrochemical cells, such as batteries and fuel cells.
Analytical Chemistry:Balanced redox reactions are used in titrations and other analytical techniques to determine the concentration of unknown solutions.
Industrial Processes:Balanced redox reactions are essential for understanding and controlling various industrial processes, such as electroplating and the production of chemicals.
FAQ Compilation: Balance The Redox Reaction By Inserting The Appropriate Coefficients.
What is the significance of balancing redox reactions?
Balancing redox reactions is crucial for understanding the stoichiometry of chemical reactions, ensuring that the number of electrons lost by reducing agents equals the number of electrons gained by oxidizing agents.
How does inserting appropriate coefficients aid in balancing redox reactions?
Inserting appropriate coefficients in front of reactants and products ensures that the number of atoms of each element is the same on both sides of the reaction, satisfying the law of conservation of mass.
What are common mistakes to avoid when balancing redox reactions?
Common mistakes include overlooking changes in oxidation states, incorrectly assigning coefficients, and failing to account for spectator ions.